34. Synergistic LMCT and Ni Catalysis for Methylative Cross-Coupling Using tert-Butanol: Modulating Radical Pathways via Selective Bond Homolysis
J. Am. Chem. Soc. 2025, 147, 14785−14796. DOI: 10.1021/jacs.5c03711
Lingfei Duan, Yunzhi Lin, Qing An, Zhiwei Zuo*
Abstract
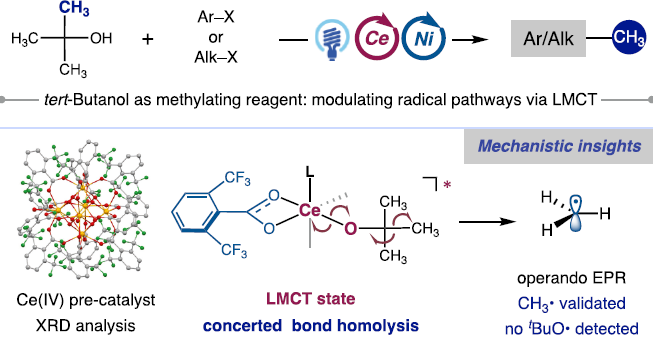
Ligand-to-metal charge transfer (LMCT) excitation has emerged as a potent strategy for the selective generation of heteroatom-centered radicals, yet its full potential in modulating open-shell radical pathways remains underexplored. Here, we present a photocatalytic methylative cross-coupling reaction that capitalizes on the synergistic interplay between LMCT and Ni catalysis, nabling the use of tert-butanol as an efficient and benign methylating reagent. The electron-deficient ligand 2,6-ditrifluoromethyl benzoate facilitates Ce(IV)-mediated bond scission of tert-butanol, generating a methyl radical that is subsequently captured by the Ni catalytic cycle to form C−CH3 bonds. Under mild reaction conditions, this strategy affords efficient methylation of sp3 carbons adjacent to carbonyls and sp2 centers, demonstrating broad functional group tolerance and applicability in late-stage functionalization of bioactive molecules. Additionally, trideuteromethylative coupling can be facilely achieved using commercial tert-butanol-d10. This approach circumvents the need for traditional tert-butoxy radical precursors, such as peroxides, while strategically modulating the radical pathway to favor β-scissionand suppress unwanted tert-butoxy radical formation in solution. Mechanistic studies reveal that the benzoate ligand plays a crucialrole in enabling LMCT excitation and facilitating methyl radical generation, supporting a concerted Ce−OR and β-C−C bondhomolysis mechanism, further evidenced by the modulation of regioselectivity in alkoxy radical-mediated β-scission.