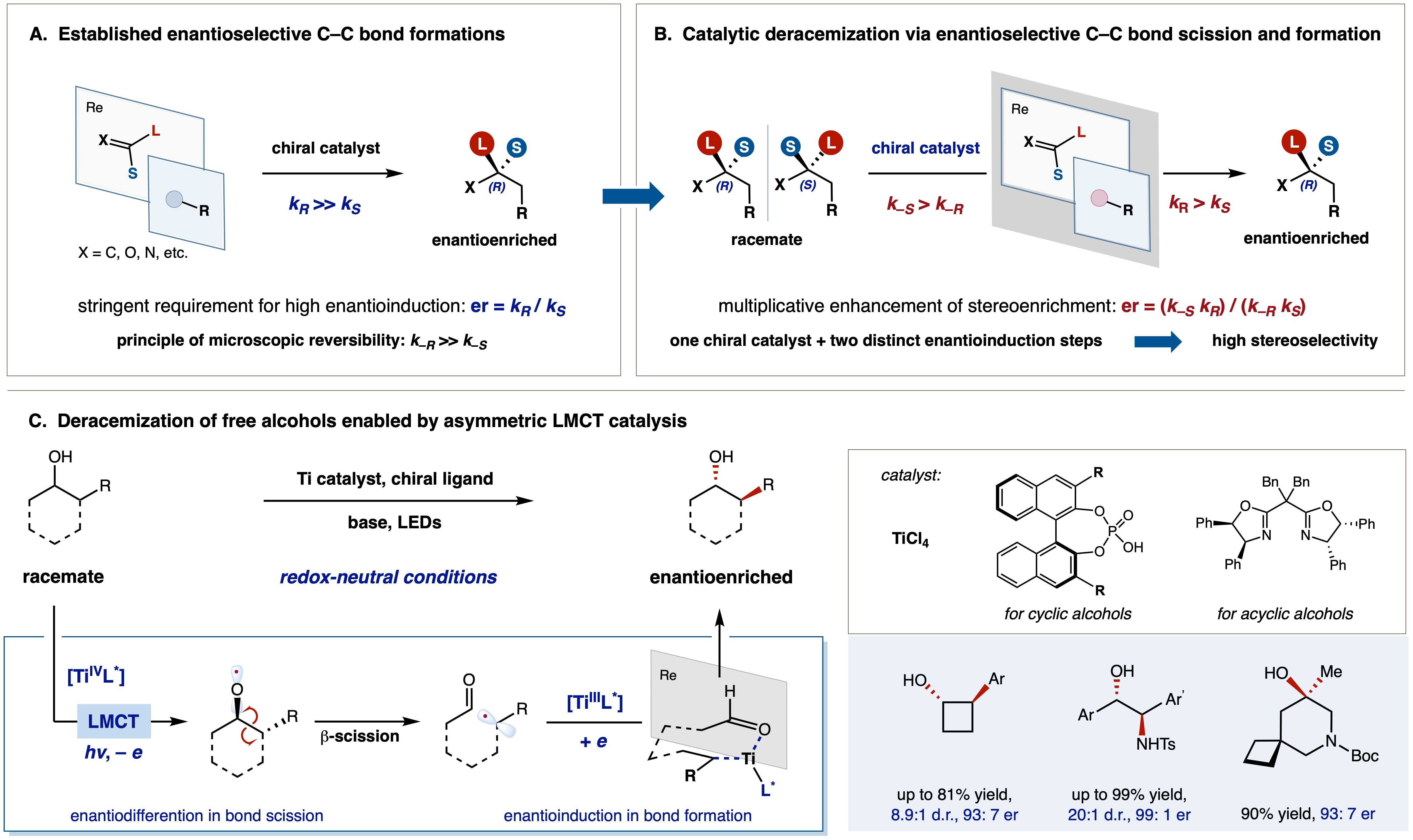
26. Multiplicative enhancement of stereoenrichment by a single catalyst for deracemization of alcohols
Science, 2023, 382, 458–464. DOI: 10.1126/science.adj0040
Abstract
Stereochemical enrichment of a racemic mixture by deracemization must overcome unfavorable entropic effects as well as the principle of microscopic reversibility; recently, photochemical reaction pathways unveiled by the energetic input of light have led to innovations toward this end, most often by ablation of a stereogenic C(sp3)–H bond. We report a photochemically driven deracemization protocol in which a single chiral catalyst effects two mechanistically different steps, C–C bond cleavage and C–C bond formation, to achieve multiplicative enhancement of stereoinduction, which leads to high levels of stereoselectivity. Ligand-to-metal charge transfer excitation of a titanium catalyst coordinated by a chiral phosphoric acid or bisoxazoline efficiently enriches racemic alcohols that feature adjacent and fully substituted stereogenic centers to enantiomeric ratios up to 99:1. Mechanistic investigations support a pathway of sequential radical-mediated bond scission and bond formation through a common prochiral intermediate and reveal that, although the overall stereoenrichment is high, the selectivity in each individual step is moderate.