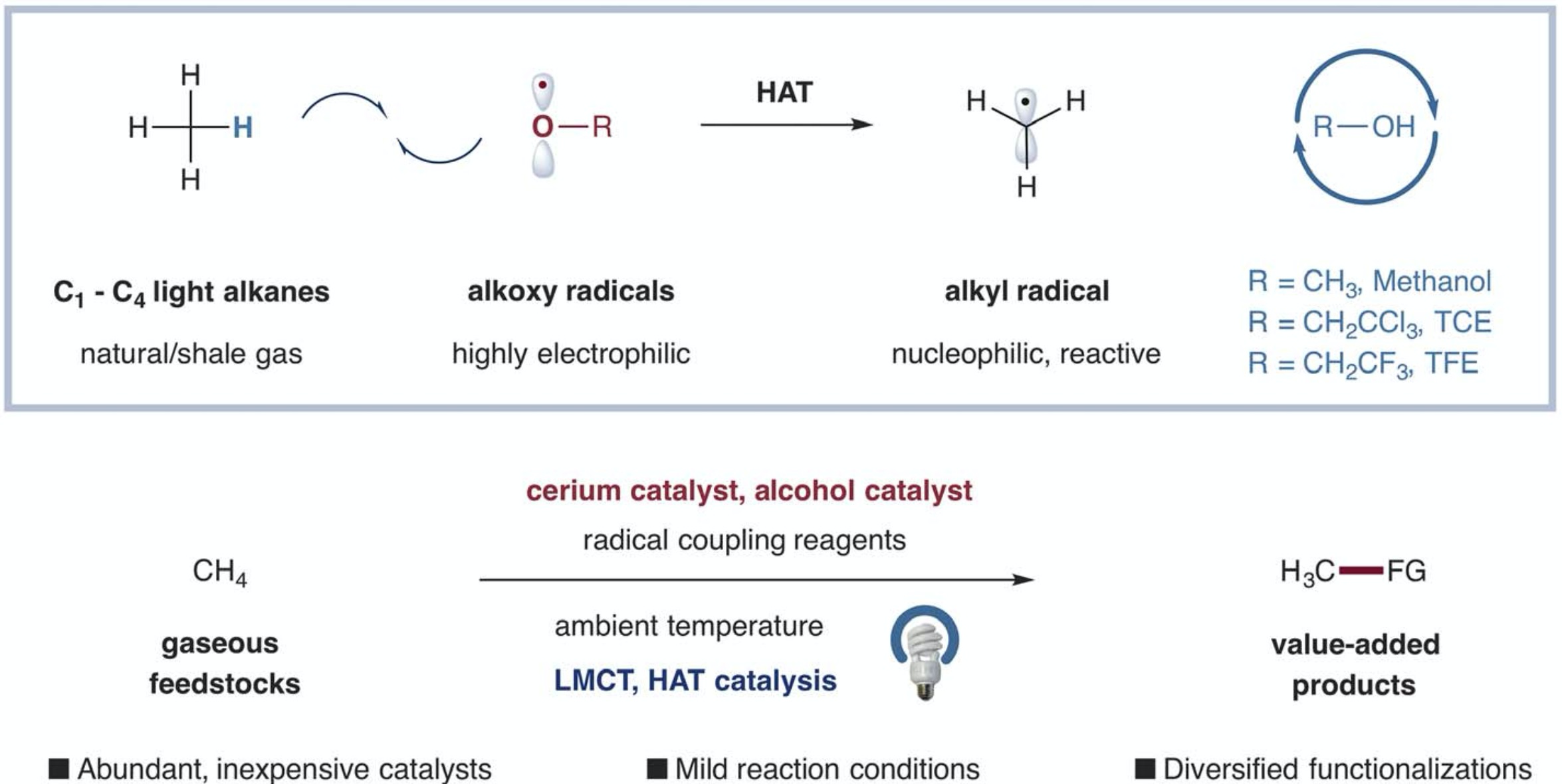
11. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis
Science, 2018, 361, 668–672. DOI: 10.1126/science.aat9750
Anhua Hu‡, Jing-jing Guo‡, Hui Pan, and Zhiwei Zuo*
Abstract
The methane, ethane, and propane in natural gas are mostly inert under ambient conditions. Mainly they are burned to produce heat. Hu et al. show that a simple cerium salt paired with an alcohol can catalytically transform these and other simple hydrocarbons into reactive radicals at room temperature (see the Perspective by Kanai). The reactions rely on light to photolytically cleave cerium alkoxide bonds, producing alkoxy radicals that strip H atoms from the hydrocarbons and regenerate the alcohol. The resultant alkyl radicals readily add to azo compounds, olefins, and aromatics.